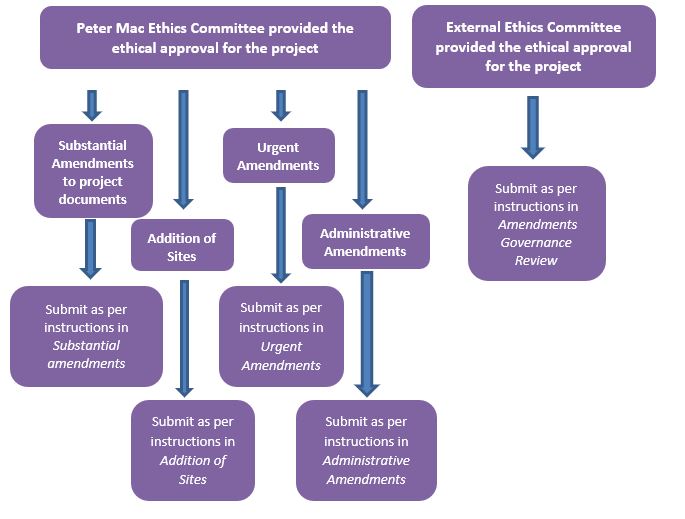
If our Ethics Committee provides the ethical review for a project, a committee or review panel must review substantial amendments to approved project documents. We provide upcoming submission due dates at Submission and Meeting Dates.
The Human Research Ethics and Governance office can review and approve other amendments and you can submit these at any time. Examples include:
- Addition of sites
- Addition or change of study personnel
- Update to contact or sponsor details
- Correction of minor errors
- Increase in accrual
If the reviewing Human Research Ethics Committee (reviewing HREC) is an external Ethics Committee, you must submit the approved amendment for governance review. You can submit these amendments to the Research Governance Office at any time.
Amendment review pathway summary
Amendments for ethical review
SOP004 Monitoring Ongoing Research describes the requirements for submitting Amendments to our Ethics Committee.
Ethics and Governance Contact
- Email:
This email address is being protected from spambots. You need JavaScript enabled to view it. - Ethics Phone: (03) 8559 7540
- Governance Phone: (03) 8559 7545